After many years of studies, the research team of the Immunology Laboratory of the Department of Biology at the University of Crete, under the leadership of Prof. Irene Athanassakis in collaboration with Dr.’s Emmanuel Stratakis research team at the Institute of Electronic Structure and Laser at FORTH, developed a system of personalized implantable vaccines, which ensures the optimal response of each organism without side effects.
Starting with the development of vaccines against simple substances (antigens), their studies were successfully extended to infectious microorganisms (Salmonella typhimurium) and then to cancer immunotherapy. The initial findings were published in the international journal Vaccine[1], while the following work was presented at the European Congress of Immunology, in September 2015. In fact, at this Conference, their work entitled “Transplantable immune modulation in response to autologous cancer cells” (PD26.13), was honored with the “EFIS 2015 Travel Grant award”. Recently this technology has been applied to experimental models in sepsis immunotherapy[2], as well as a vaccine against SARS-CoV-2.
The promising new technology that was developed at the University of Crete has already been granted two patents to the OBI (Industrial Property Organization)[3] and the patent for the application of the technology against COVID 19 will be filed soon.
The technology in a picture ….
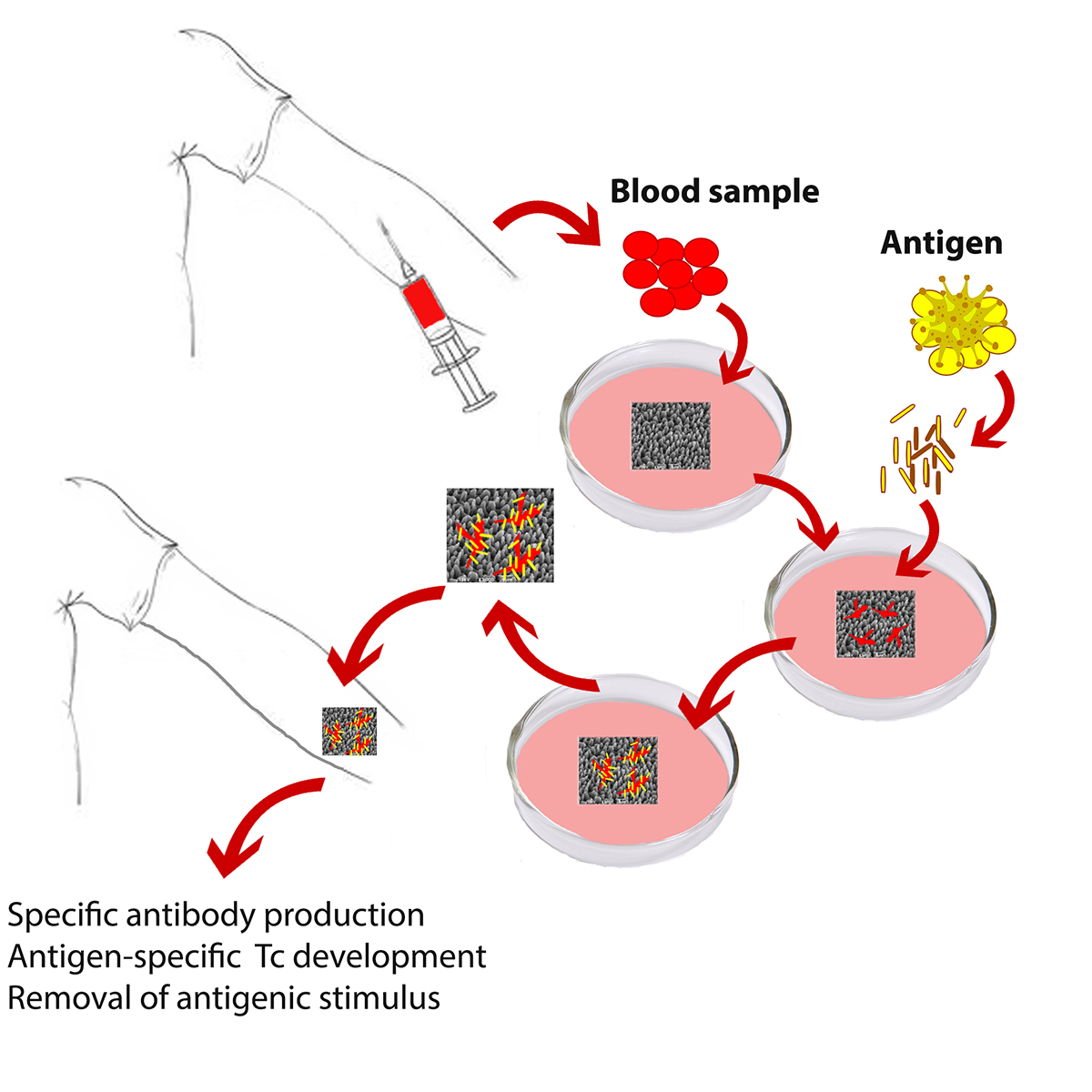
The application of this new technology in the cancer immunotherapy focuses on the personalized activation of the patient’s immune system against the specific type of tumor of the patient (Fig. 1). The appropriate immune cells are being isolated from the patient’s blood. These are allowed to physically attach on a 3D surface. Fragments of biopsy-derived tumor cells are added to the culture. The cells attached on the 3D surfaces take up the fragments and they naturally process them for presentation to the immune system. The “activated” surfaces once implanted to the patient, activate the immune system to fight the tumor cells.
The science behind the technology
The development of fully effective and without side effects vaccines is an enigmatic and open topic in research.
“First generation” vaccines mainly deal with the weakening of a microorganism. These are capable of inducing specific T and B cell responses. However, although they are more effective, there is a significant risk of infection. The pathogen can return to an infectious state and cause the disease. The need to develop more effective vaccines with low side effects has led to the “second generation” of vaccines. These are vaccines that do not use the whole microorganism, but specific proteins or parts of its proteins. Although, these vaccines are able to induce of a specific antibody production, they need the simultaneous administration of adjuvants for non-specific immune stimulation of the organism. Unfortunately, these adjuvants display non-specific side effects and are not yet effectively controlled. Vaccine safety is a serious issue puzzling the World Health Organization. The fact that vaccine safety can only be evaluated after massive immunizations raises serious bioethical issues. Although such vaccines have successfully eliminated pathogen’s infectivity, the adjuvant-mediated non-specific effects are still depriving the required safety. “Third generation” vaccines, or DNA / mRNA vaccines, follow a similar rationale to second-generation vaccines, except that they do not provide the protein itself to the organism, but go one or two steps ahead of the protein production mechanism, namely they provide mRNA that will be translated into protein, or DNA that will be transcribed into mRNA and then translated into protein. An additional disadvantage of these vaccines is that the amount of protein produced in each organism is not controlled.
Another important issue to consider when developing a vaccine is the exclusivity of each individual in terms of histocompatibility (which is also responsible for transplant rejection). Histocompatibility is represented by two main types of proteins, class I and class II proteins. These proteins are necessary for the development of an immune response, since they form stable complexes with some small fragments (antigenic epitopes) of the pathogen. Thus, each organism fights the respective pathogen, in its own particular way, which depends on the personal histocompatibility. We understand, therefore, that the larger the microorganism that infects the organism, the greater the chances of finding a pathogenic part that matches its histocompatibility, and therefore fighting the pathogen. This is why first generation vaccines are more effective. In contrast, in the second generation of vaccines, this possibility is limited due to the lower number of antigenic epitopes. In this case, there are people who fail to develop an immune response and are led to apathy.
To overcome these difficulties, the original idea towards the development of the technology described here was to find a system, in which the initial activation of an individual’s immune system by the microorganism would be applied to the individual’s own cells, but outside organism (in vitro). This avoids infectivity. Then, the implantation of this bio-system (in vivo) could activate the whole organism. The implementation of this idea leads to the personalized vaccination, while at the same time eliminating all side effects due to the non-specific stimulation of the adjuvants, since the system used causes the necessary mild stimulation for the initiation of the immune response.
The proposed implantable structures consist of three-dimensional silicon surfaces microstructured using an ultrafast laser beam. The proposed laser-structured silicon surface allows controllable and highly repetitive construction outcome.
The study
The choice of the appropriate surface roughness and chemistry was based on the adhesion of the antigen-presenting cells and their ability to provide specific antibody and growth factor production upon antigen stimulation and addition of the required immune cell populations (Fig. 2).
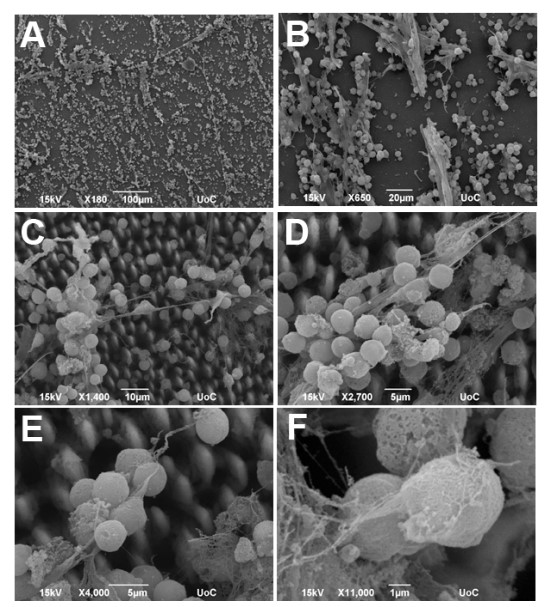
Figure 2: Co-cultures of macrophages and T cells growing on the surface of silicon scaffolds. The large numbers of secretory bodies that decorate the tips of the scaffolds were made visible by scanning electron microscopy. The images show successive magnifications of the culture, ranging from x180 (A) to X11,000 (F).
The transfer of the in vitro successful immunization in vivo had to consider two additional factors: the first was whether these silicon surfaces could provide some immunostimulation to the organism in order to avoid adjuvants and the second was whether they could induce antigen-specific antibody production.
Indeed subcutaneous implantation of the structures could cause immunostimulation like common adjuvants, while also effectively inducing specific antibody production. The implantable vaccine technology has been successfully applied in vivo in mice against various antigenic stimuli, including human serum albumin (HSA), the bacterial strain Salmonella typhimurium, SARS-CoV-2, as well as autologous tumor antigens, while following appropriate manipulations, the application of such technology could also reverse sepsis phenotype in experimental mouse models.
In the context of cancer immunotherapy, the antigenic stimulus is tumor cell lysate from the patient’s biopsy. But since tumor cells are part of the same organism, how is it possible for the organism to fight its own cells? Indeed, one of the mechanisms used by tumor cells to escape immune attack is suppression of the immune system. Thus, tumor cells grow undisturbed. Tumor cells are characterized by loss of controlled cell division, creation of “neo-antigens” through point mutations, insertions / deletions, inversions, etc., all of which create favorable conditions for growth. During the last decade, these “neo-antigens” have been the target of cancer vaccines, but with very limited success. The exclusivity of tumor cells of each patient makes it difficult to develop universal vaccines, indicating the need to develop personalized processes.
The personalized therapy must be adapted not only to the particular type of tumor cells of each individual, but also the particular needs of the respective organism to develop an immune response against tumor cells. As already mentioned, the design of a vaccine is a very delicate procedure, because it can lead to opposite results. So, direct vaccination of a patient with his own tumor cells would risk worsening his clinical picture. The personalized implantable vaccine technology pioneers in two important directions: The first is that vaccination with tumor cells of the same organism is carried out in vitro. The “antigen” is administered in cultured host cells isolated from peripheral blood, to define the optimal conditions / parameters to sensitize the immune system of the individual. The activated cells are then administered to the host, providing immediate protection. The second important element is the tumor-activated host cells are not given in a free form, because they cannot easily find their target. The activated cells adhere to specific surfaces and are provided to the host as an implant, which in turn attracts all necessary immune cells for immediate protection and destruction of tumor cells.
Thus, implantable vaccines consist of a microstructured silicon surface loaded with autologous cells activated by tumor cell lysates of the patient. This process takes place in culture. The activated scaffold is then implanted subcutaneously. Only one week after implantation, the development of a specific immune response against the tumor is observed, while the improvement of the clinical picture of the experimental animals is spectacular.
This approach is a personalized vaccination, which eliminates all non-specific side effects due to adjuvants, since the structure itself causes the necessary stimulation for the initiation of the immune response. The proposed laser-structured silicon scaffolds have significant advantages as silicon is flexible and independent material, easily adaptable and scalable through parallel processing, allowing the controlled and high resolution creation of three-dimensional structures.
The proposed technology combines in vitro and in vivo development of specific immune system stimulation. This approach overcomes the need for additives, ensures the best choice of antigenic peptides for each individual and avoids pathogens and infectious agents, since the antigen is only provided in culture (in vitro).
The application of this technology in cancer immunotherapy focuses on the personalized activation of the patient’s immune system against the specific own type of cancer. A small blood sample is used to isolate the antigen-presenting cells of the patient, which are cultured on the 3D surface in the presence of biopsy-derived tumor cell extract that serves as the antigenic stimulus for cell activation. Tumor-specific antigens that develop in cancer cells are unique to each individual and are used to activate the specific defense of each patient by producing antibodies, but mainly by activating cytotoxic T cells to neutralize malignant target cells (Fig. 3).
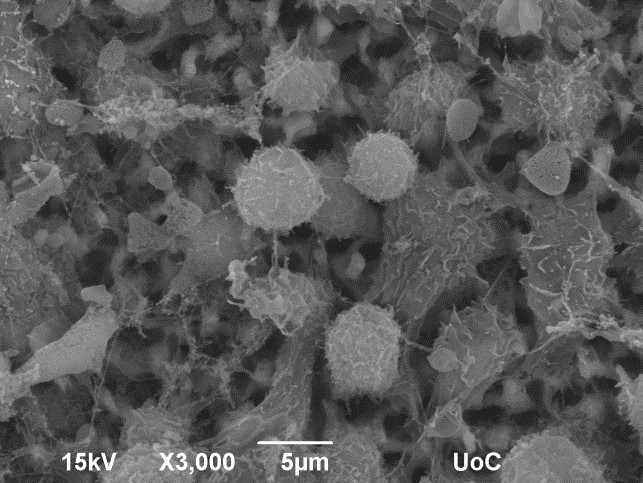
Figure 3: Lymphocytes are successfully attracted by tumor extract-activated macrophages on top of the scaffold
The application of Personalized Implantable Vaccine technology opens new horizons of great importance, both in research and in therapy. Taking advantage of natural mechanisms, we will now be able to deal with the serious health problems with simple, costless, but specialized approaches.
[1] Zerva, I., Simitzi, C., Siakouli-Galanopoulou, A., Ranella, A., Stratakis, E., Fotakis, C., Athanassakis I. Implantable vaccines: In vitro antigen presentation enables in vivo immune response. Vaccine 33: 3142–3149, 2015. http://dx.doi.org/10.1016/j.vaccine.2015.04.017
Zerva, I.,Katsoni, E., Simitzi, C., Stratakis, E., Athanassakis, I. Laser micro-structured Si scaffold implantable vaccines against Salmonella Typhimurium. Vaccine 37: 2249-2257 (2019) doi.org/10.1016/j.vaccine.2019.02.080
[2] Zerva I, Bakela K, Athanassakis I. Immunotherapy-on-chip against an experimental sepsis model. Inflammation 2021 (in press)
[3] Athanassakis, I., Zerva, I., Simitzi, C., Ranella, A., Stratakis E., Personalized implantable vaccines using antigen pre-activated monocytes (Εξατομικευμένα εμφυτεύσιμα εμβόλια με αντιγονικά διεγερμένα μονοκύτταρα). ΟΒΙ 20140100471, 19/9/2014 https://worldwide.espacenet.com/patent/search/family/056090633/publication/GR1008652B?q=20140100471
Athanassakis I., Zerva, I., Stratakis E.. Personalized implant against cancer. Εξατομικευμένο εμφύτευμα κατά του καρκίνου. ΟΒΙ 20190100297


 Ελληνικά
Ελληνικά